

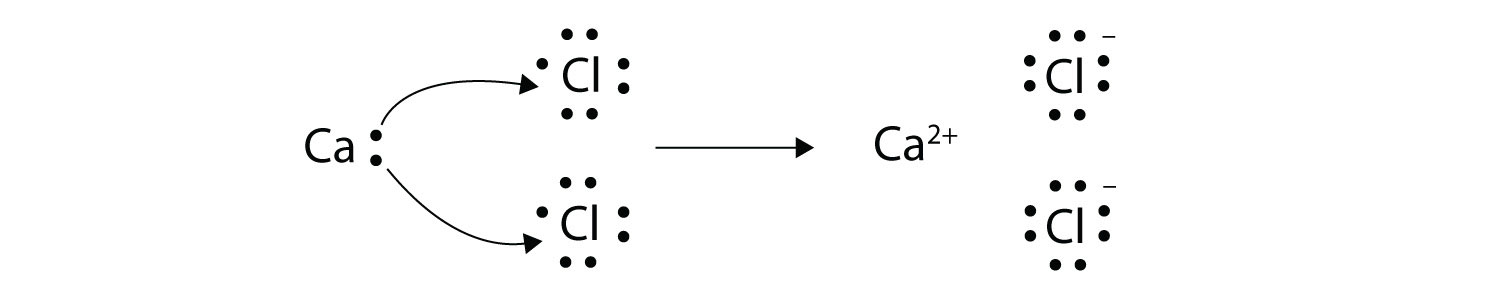
This is not observed in complex organic compounds like sugar (C 12H 22O 11). According to Dalton, atoms of different elements combine in simple whole number ratios to form compounds.This has been proven wrong in certain cases: argon and calcium atoms each have an atomic mass of 40 amu.

You can see the different atoms represented by colour. Sometimes the atoms are all from the same element. To form a compound - on the left - you need 2 or more different atoms to bond together.
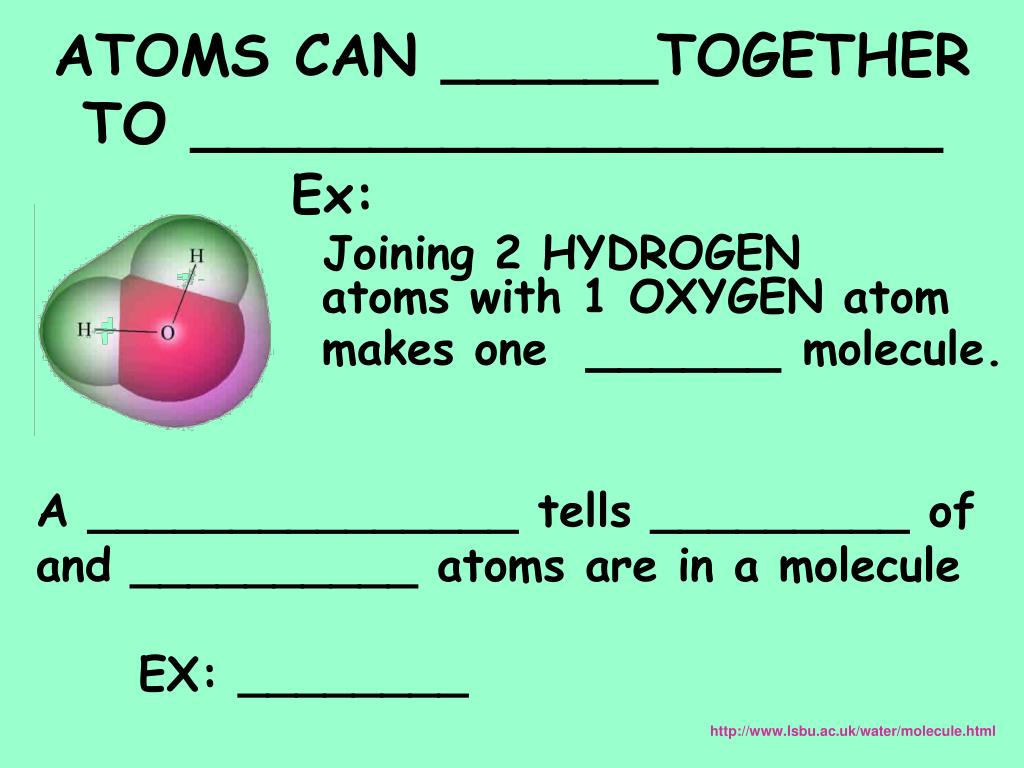
When two atoms share electrons between them they are locked together (bonded) by that sharing. According to Dalton, the atoms of same element are similar in all respects. Atoms come together to form molecules because of their electrons.However an atom is the smallest particle that takes part in chemical reactions. Elements join together to form compounds. Molecules join together to form elements. The indivisibility of an atom was proved wrong: an atom can be further subdivided into protons, neutrons and electrons. Atoms join together to form what Well, they can form molecules.His findings were based on experiments and the laws of chemical combination. In general, bonding is a chemical change that occurs during chemical. Each individual atom consists of smaller particlesnamely, electrons and nuclei. The process of two or more atoms joining together to form a molecule is called bonding. Molecules, in turn, are composed of atoms joined by chemical bonds that are more difficult to break. John Dalton, a British school teacher, published his theory about atoms in 1808. Most matter consists of an agglomeration of molecules, which can be separated relatively easily. To help show this three-dimensional shape even more accurately, we can rely on space-filling models as well as ball-and-stick models.\) We will discuss the significance of these electrons at the end of this section. So I decomposed the clusters, and once I had them as single atoms, I wrote my name. As that was what I was studying, I decided to try to move some clusters that appear in the natural sample and see what they are made of. The two dots above nitrogen indicate a lone pair of electrons that are not involved in any covalent bond. I used Mn atoms because Im studying the growth of Mn on Ir and its magnetism at this resolution, which is quite interesting. However, in the more detailed structural formula on the right, we have a dashed line to indicate that the rightmost hydrogen atom is sitting behind the plane of the screen, while the bold wedge indicates that the center hydrogen is sitting out in front of the plane of the screen. Calculate the number of atoms of each element present in 3.96 g of water. Covalent bonds are formed when the atoms share electrons in order to usually attain a more stable arrangement although there are some arrangements where the molecule is more unstable than the individual atoms. This would be a cleaner, safer, more efficient and more abundant source of power than. It's the same type of reaction that powers hydrogen bombs and the sun. Within these molecules the atoms are held together by various chemical bonds.
In a fusion reactor, hydrogen atoms come together to form helium atoms, neutrons and vast amounts of energy. Atoms join or bond together to form molecules. In the structural formula to the left, we are only seeing a two-dimensional approximation of this molecule. Molecules are held together by covalent bonds between the atoms. In nuclear fusion, you get energy when two atoms join together to form one. Keep in mind, however, that atoms and molecules, just like everything else in the universe, exist in three dimensions-they have length and width, as well as depth. From both of these structural formulas, we can see that the central nitrogen atom is connected to each hydrogen atom by a single covalent bond.


 0 kommentar(er)
0 kommentar(er)
